
NONMEM 3 COMPARTMENT SERIAL
Serial or parallel absorption from two or more sites.One or more zero-order and/or first-order absorption processes.With this basic compartmental model structure, a variety of absorption processes can be described, including: Figure 9.1 depicts the schematic diagram of a model that can be used to describe these simultaneous processes, where the variable F z is the fraction of the dose absorbed by the first-order rate, 1 – F z is the fraction of the dose absorbed by the zero-order rate, V is the volume of distribution in the body, and k is the first-order elimination rate constant. One way to describe this model is to describe an absorption, or depot, compartment for each rate process and estimate the fraction absorbed through each process. The authors describe a variety of models that were tested in the PK model development process for alpha interferon, one of which described the PK properties with a one-compartment model disposition with simultaneous first-order and zero-order absorption without a lag time.
NONMEM 3 COMPARTMENT CODE
(1999) provide an interesting example of a PK scenario that we will use to demonstrate various ways to code user-written models. For the analysis of pharmacodynamic data, user-written models are generally required except in the simplest cases.Ĭhatelut et al. Recirculation, transit compartments, and nonlinear elimination or transfer processes are other settings in which the need for more complex PK models is frequently encountered. For pharmacokinetic (PK) analysis, perhaps the most common need for user-written models is to describe the complex nature of drug absorption rather than an unusual arrangement or number of compartments in a model. We will focus on the expression of these user-written models using PREDPP, including models not specified with the specific ADVANs, which must be defined specifically by the analyst using the general ADVANs (5, 6, 7, 8, 9, and 13). The expression user-written models in this chapter refers to control files in which the complete mathematical structure of the model must be specified by the analyst. The use of any model in NONMEM requires a user-written control file that includes elements such as code to call the dataset, the names of the parameters to be estimated, specification of the fixed- and random-effect parameter structures, and initialization of parameter values. Our use of the expression user-written model does not simply mean writing a control file. The PRED and PREDPP routines of NONMEM provide flexibility to describe complex models and are not limited to the set of prebuilt models provided in the specific ADVAN routines (ADVAN1, 2, 3, 4, 10, 11, and 12) or to a limited set of numbers of compartments or functional forms for rate and transfer processes.
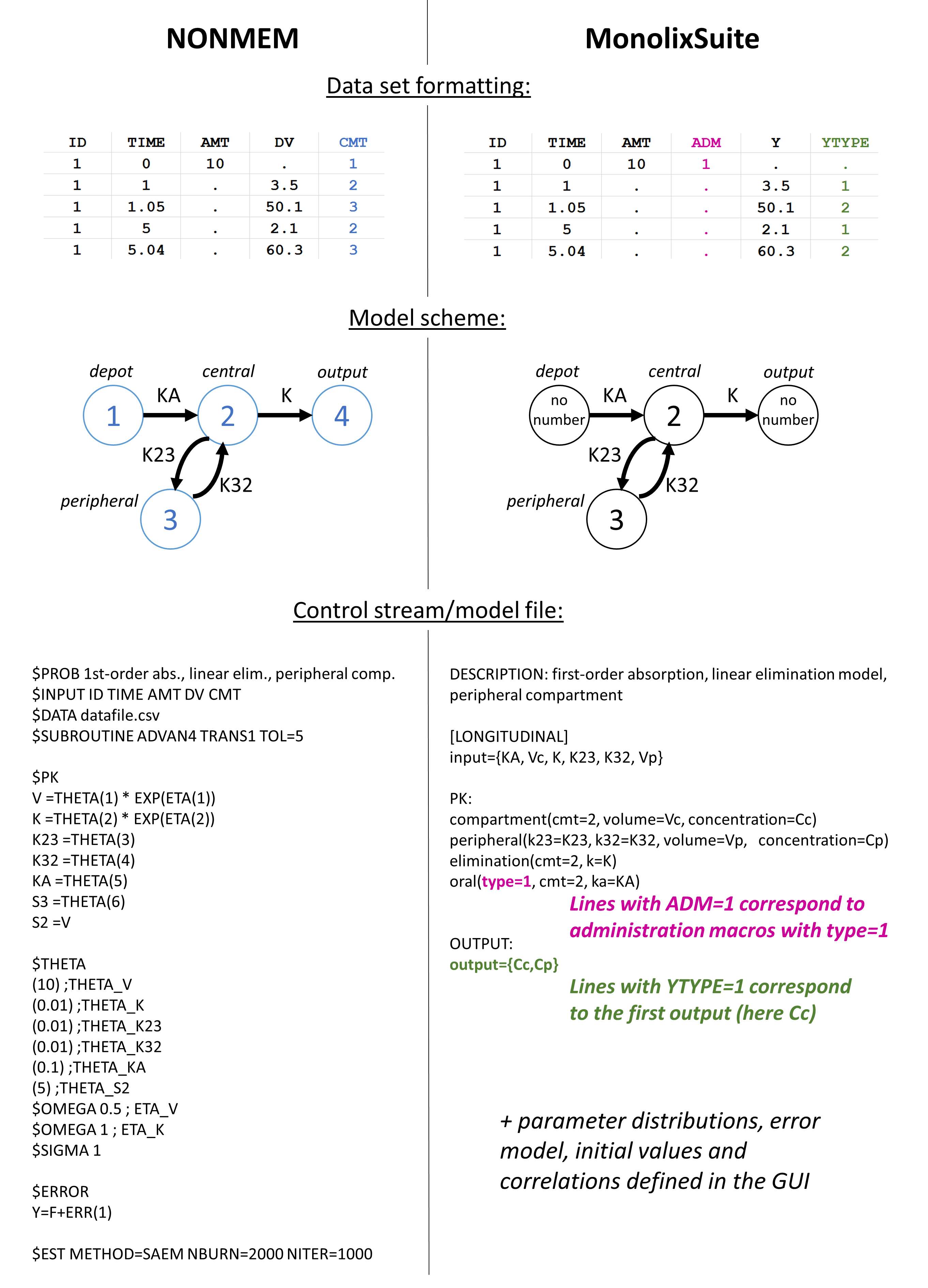
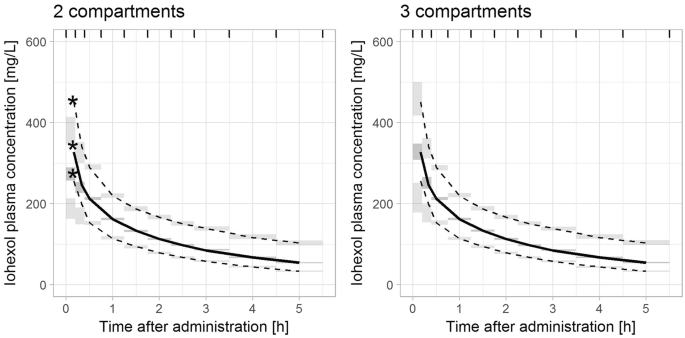
Nonlinear mixed effect models are useful for the construction of an immense variety of pharmacometric models.


 0 kommentar(er)
0 kommentar(er)
